High-Throughput Experimentation Enables One-Pot MAb Reduction/Conjugation
Explore how high-throughput experimental design delivers rapid, resource-efficient insights into mAb reduction and conjugation using phosphine-based reductants
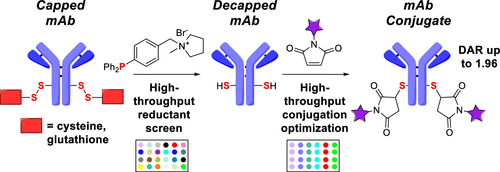
This manuscript describes the detailed evaluation of more than 40 phosphine reductants via automated and nonautomated high-throughput experimentation approaches with the goal of identifying selective reductants for cleaving the disulfide bonds of capped, engineered cysteines in a proprietary monoclonal antibody (mAb). As a point of reference, this study included phosphines that have previously been documented in the literature [4-diphenylphosphino benzoic acid (DPPBA), tris(3-sulfophenyl)phosphine (TSPP), and 3-(diphenylphosphino)benzenesulfonate (diPPBS)]; however, all known reductants showed the formation of undesired side products upon reduction (detectable by IEX), especially at higher phosphine loadings. The high-throughput study also revealed several phosphines with potential for selective reduction that had not been previously studied for this type of transformation. These initial hits were further evaluated with regard to the phosphine/mAb ratio, solubility in aqueous media, and air oxidation behavior. The best phosphine identified (1-(4-(diphenylphosphanyl)benzyl)-1-methylpyrrolidin-1-ium bromide (P10)) was then employed in a sequence of high-throughput studies that established efficient one-pot reduction/conjugation reaction conditions. Overall, the work detailed herein demonstrates how high-throughput experimental design enables rapid and resource-sparing insights into mAb reduction and conjugation reactivity with phosphine-based reductants.





